CIP | Clean in Place
Clean-in-place (CIP) systems are widely used in the pharmaceutical industry to ensure the cleaning of equipment and surfaces during the manufacturing process, using a combination of chemical and mechanical processes to remove impurities and residues that may compromise product quality.
Our CIP systems can be fixed or mobile and are designed to be fully automated and programmed to clean equipment and/or complete processing lines. During this process the cleaning solution circulates through the equipment, removing any residues and impurities and is then drained away. This process is repeated several times, ensuring all surfaces are thoroughly cleaned.
CIP systems are essential for not only maintaining the high level of cleanliness required in the pharmaceutical industry, but also for increasing the efficiency and quality of your production by reducing downtime for cleaning activities.

SIP | Sterilization in Place
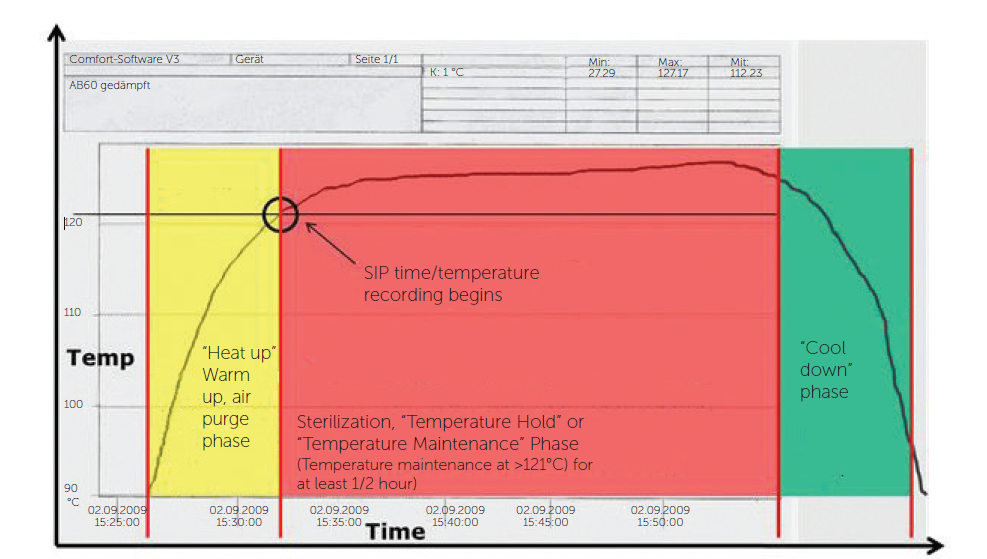
Another system widely used in the pharmaceutical industry is the SIP (Sterilization-In-Place). These systems are used to sterilize equipment and pipes, minimising the risk of contamination.
Through a thermoregulation system, it is possible to heat the tank internally for a period of time, up to the sterilization temperature – 121ºC. At this temperature all microorganisms are extinguished, ensuring the sterility and cleanliness of the equipment, an essential factor in the pharmaceutical industry.
Our SIP systems allow complete sterilization of the equipment and tubing, without the need for disassembly, saving time and ensuring the effectiveness and safety of the final product to be produced.









