Quality
We work every day to achieve exceptional quality.
With over 20 years of experience, knowledge and expertise in the pharmaceutical industry, we work to
maintain maximum quality control and ensure solutions that meet the highest standards of our customers
and the industry.
QUALITY POLICY
CERTIFICATION
All FSSE® systems involve careful checking of functionality and design to ensure that the end product meets all regulated requirements. All our processes are certified according to:
- TUV Rheinland Portugal® certification organization based on ISO 9001:2015.

| CERTIFIED WELDING
PROCESSES
Based on the requirements of EN ISO 9606 – 1 and EN ISO 15614-1, our team has the qualifications and means to master a wide range of welding processes.

| ADOPTED STANDARDS
All our services, processes and products are carried out following a strict set of external standards, which include:
- EN13445
- PED | Pressure Equipment Directive (2014/68/EU)
- cGMP | Current Good Manufacturing Practice
- cGEP | Current Good Engineering Practice
- FDA
- GAMP | Good Automated Manufacturing Practice
- ATEX
- Machinery Directive
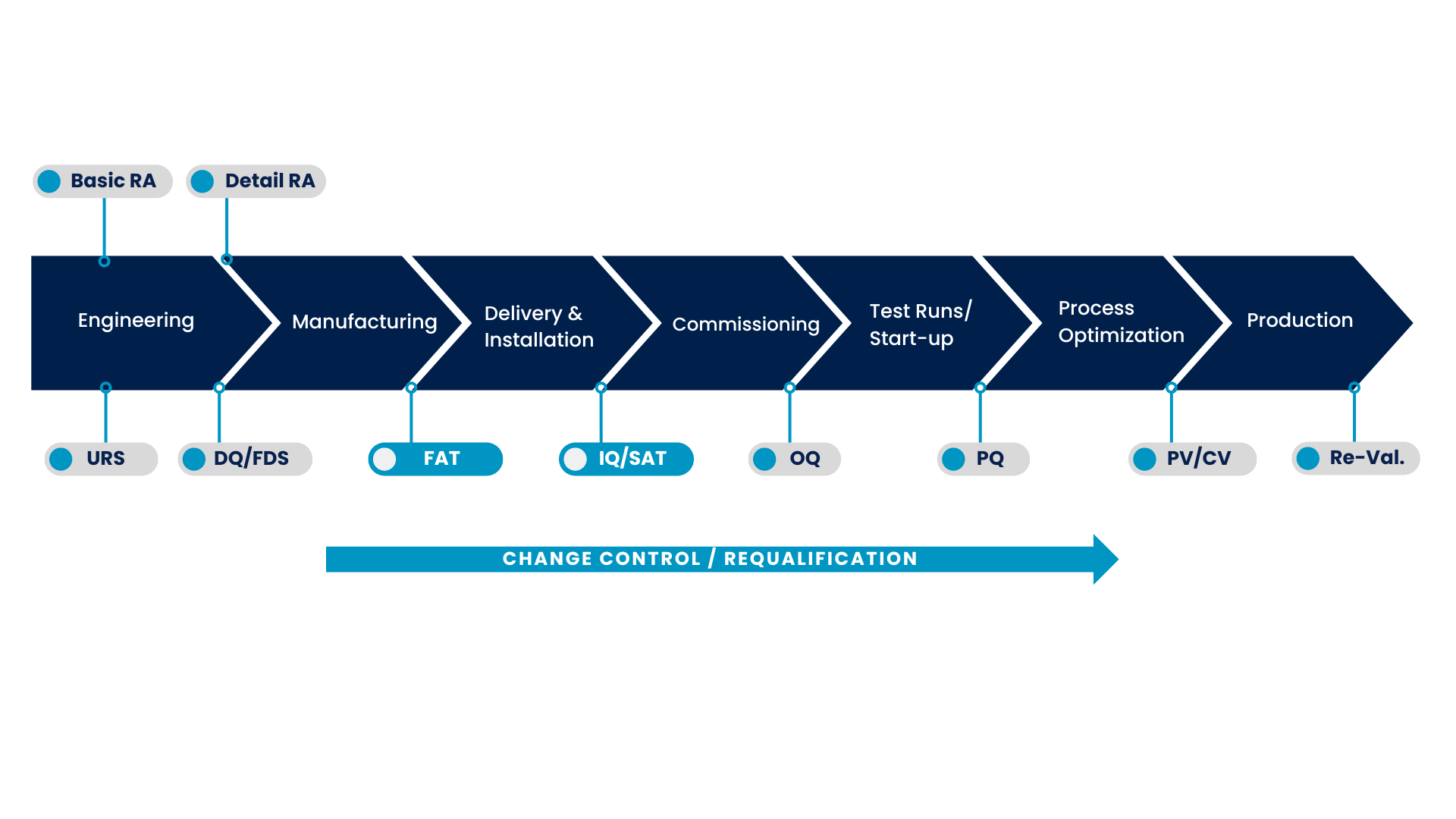
VALIDATION, ACCEPTANCE AND QUALIFICATION
VALIDATION
The objective of validation is to ensure that processes are reliable and capable of producing high-quality products, particularly in industries focused on human health such as the pharmaceutical sector.
For this reason, we follow GMP/GAMP principles on a daily basis, documenting the reliability of all our processes and systems and verifying that they operate in accordance with the prescribed specifications.


ACCEPTANCE
When the process of building your equipment is completed, several tests are performed on the new equipment.
The first test is the Factory Acceptance Test (FAT), which takes place on the FSSE ® facilities, together with the client, where different real-world scenarios are simulated. This allows any deviations or faults to be identified, which will be quickly rectified before they have a significant impact on the client’s process.
Once the FAT is completed, the system is then transported to the customer’s premises and integrated with other systems and networks. An on-site SAT-Site Acceptance Test is subsequently performed, verifying the connection and interaction of the system with local facilities such as electricity, water, or CIP cleaning systems.
QUALIFICATION
Throughout our process, we establish qualification protocols that allow us to assess and validate the reliability and usability of your equipment.
- Before production of the equipment, during Design Qualification (DQ), the design is meticulously assessed and approved.
- Once the equipment has been installed, Installation Qualification (IQ) assesses its technical specifications and confirms that the installation has been carried out correctly and according to URS.
- During Operational Qualification (OQ), the installation is tested to ensure that all components of the product work as intended.
- Finally, Performance Qualification (PQ) evaluates the quality of the system results through multiple executions of the process to produce the desired application.